This study guide will enable you to learn more about cholera, its risk factors, clinical manifestation, treatment, nursing diagnosis, nursing interventions, and nursing management.
Cholera which continues to be a threat to public health, usually affects individuals who has travel to or live in places with poor sanitation and lack of safe drinking water. This disease is also closely related with poverty, overpopulation, lack of safe disposal of excreta, and unhygienic practices during food preparation, handling and storage.
What is Cholera?
Cholera is an acute diarrhoeal disease caused by Vibrio cholerae.
- Records from Hippocrates (460-377 BCE) and the Indian peninsula describe an illness that might have been cholera.
- Although not the first description, the discovery of the cholera organism is credited to German bacteriologist Robert Koch, who independently identified V cholerae in 1883 during an outbreak in Egypt; the genus name refers to the fact that the organism appears to vibrate when moving.
- The hallmark of the disease is profuse secretory diarrhea.
- Cholera can be endemic, epidemic, or pandemic.
Pathophysiology
Cholera, caused by the bacteria Vibrio cholerae, is a comma-shaped, gram-negative aerobic or facultatively anaerobic bacillus that varies in size from 1-3 µm in length by 0.5-0.8 µm in diameter.
- Currently, the El Tor biotype of V cholerae O1 is the predominant cholera pathogen; organisms in both the classical and the El Tor biotypes are subdivided into serotypes according to the structure of the O antigen.
- The clinical and epidemiologic features of disease caused by V cholerae O139 are indistinguishable from those of disease caused by O1 strains; both serogroups cause clinical disease by producing an enterotoxin that promotes the secretion of fluid and electrolytes into the lumen of the small intestine.
- To reach the small intestine, however, the organism has to negotiate the normal defense mechanisms of the GI tract; because the organism is not acid-resistant, it depends on its large inoculum size to withstand gastric acidity.
- The use of antacids, histamine receptor blockers, and proton pump inhibitors increases the risk of cholera infection and predisposes patients to more severe disease as a result of reduced gastric acidity.
- Fluid loss originates in the duodenum and upper jejunum; the ileum is less affected.
- The colon is usually in a state of absorption because it is relatively insensitive to the toxin; however, the large volume of fluid produced in the upper intestine overwhelms the absorptive capacity of the lower bowel, resulting in severe diarrhea.
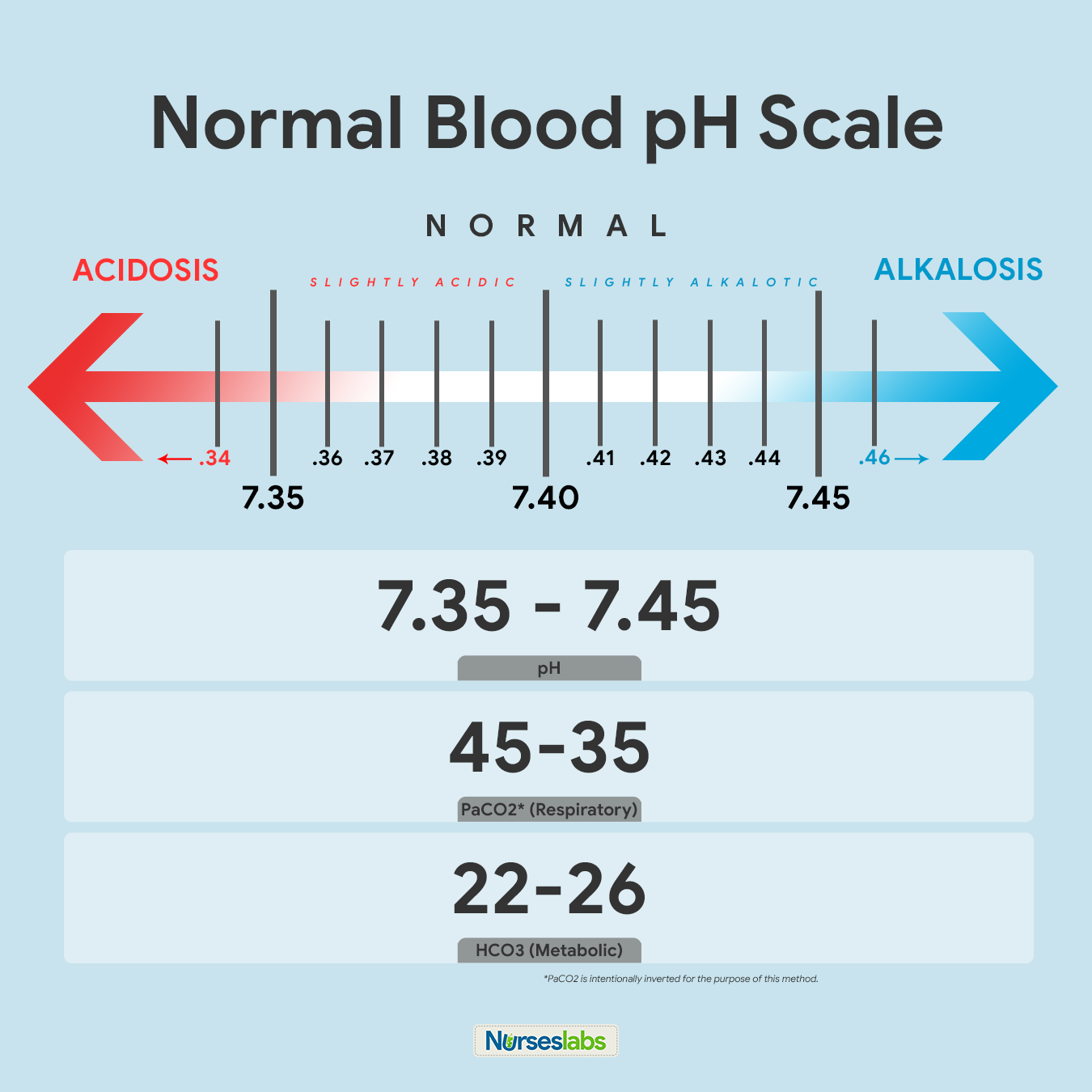
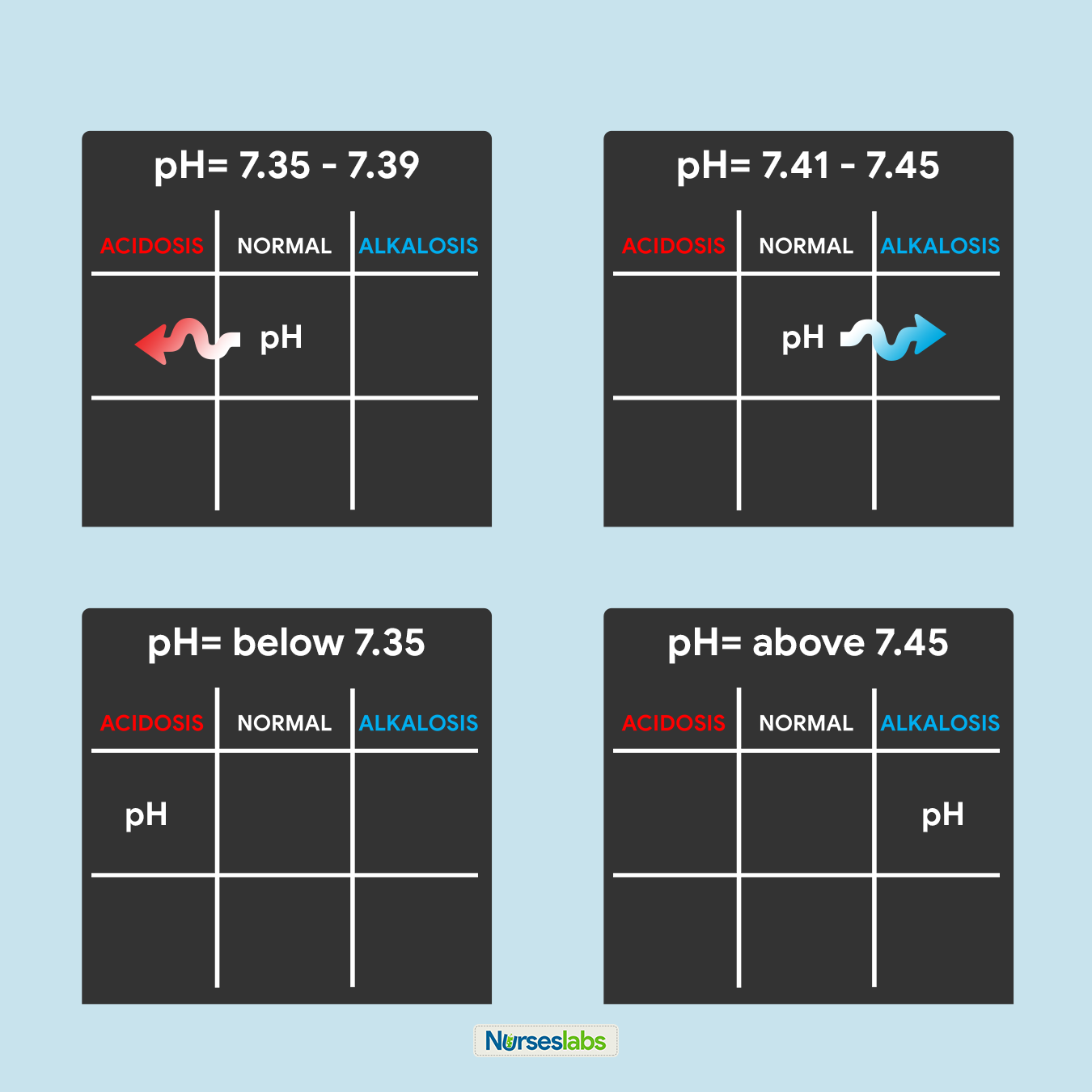
- Unless the lost fluid and electrolytes are replaced adequately, the infected person may develop shock from profound dehydration and acidosis from loss of bicarbonate.
- The enterotoxin acts locally and does not invade the intestinal wall. As a result, few neutrophils are found in the stool.
Causes
Cholera can be an endemic, epidemic, or a pandemic disease.
- Environmental factors. Primary infection in humans is incidentally acquired. Risk of primary infection is facilitated by seasonal increases in the number of organisms, possibly associated with changes in water temperature and algal blooms; secondary transmission occurs through fecal-oral spread of the organism through person-to-person contact or through contaminated water and food.
- Host factors. Malnutrition increases susceptibility to cholera. Because gastric acid can quickly render an inoculum of V cholerae noninfectious before it reaches the site of colonization in the small bowel, hydrochlorhydria or achlorhydria of any cause (including Helicobacter pylori infection, gastric surgery, vagotomy, use of H2 blockers for ulcer disease) increases susceptibility; infection rates of household contacts of cholera patients range from 20-50%. Rates are lower in areas where infection is endemic and individuals, especially adults, may have preexisting vibriocidal antibodies from previous encounters with the organism.
Statistics and Incidences
In the United States, cholera has virtually been eliminated because of improved hygiene and sanitation systems.
- The frequency of cholera among international travelers returning to the United States has averaged 1 case per 500,000 population, with a range of 0.05-3.7 cases per 100,000 population, depending on the countries visited.
- Between January 1, 1995, and December 31, 2000, 61 cases of cholera were reported in 18 states and 2 US territories.
- In 1990, fewer than 30,000 cases were reported to the WHO.
- From 2005 to 2008, 178,000-237,000 cases and 4000-6300 deaths were reported annually worldwide.
- In nonendemic areas, the incidence of infection is similar in all age groups, although adults are less likely to become symptomatic than children.
Clinical Manifestations
After a 24- to 48-hour incubation period, symptoms begin with the sudden onset of painless watery diarrhea that may quickly become voluminous and is often followed by vomiting.
- Diarrhea. Profuse watery diarrhea is a hallmark of cholera; cholera should be suspected when a patient older than 5 years develops severe dehydration from acute, severe, watery diarrhea (usually without vomiting) or in any patient older than 2 years who has acute watery diarrhea and is in an area where an outbreak of cholera has occurred.
- Vomiting. Vomiting, although a prominent manifestation, may not always be present; early in the course of the disease, vomiting is caused by decreased gastric and intestinal motility; later in the course of the disease it is more likely to result from acidemia.
- Dehydration. If untreated, the diarrhea and vomiting lead to isotonic dehydration, which can lead to acute tubular necrosis and renal failure; because the dehydration is isotonic, water loss is proportional between 3 body compartments, intracellular, intravascular, and interstitial.
Assessment and Diagnostic Findings
Definitive diagnosis is not a prerequisite for the treatment of patients with cholera.
- Stool examination. Although observed as a gram-negative organism, the characteristic motility of Vibrio species cannot be identified on a Gram stain, but it is easily seen on direct dark-field examination of the stool.
- Stool culture. V cholerae is not fastidious in nutritional requirements for growth; however, it does need an adequate buffering system if fermentable carbohydrate is present because viability is severely compromised if the pH is less than 6, often resulting in autosterilization of the culture.
- Serotyping and biotyping. Specific antisera can be used in immobilization tests; a positive immobilization test result (ie, cessation of motility of the organism) is produced only if the antiserum is specific for the Vibrio type present; the second antiserum serves as a negative control.
- Hematologic tests. Hematocrit, serum-specific gravity, and serum protein are elevated in dehydrated patients because of resulting hemoconcentration; when patients are first observed, they generally have a leukocytosis without a left shift.
- Metabolic panel. Serum sodium is usually 130-135 mmol/L, reflecting the substantial loss of sodium in the stool; serum potassium usually is normal in the acute phase of the illness, reflecting the exchange of intracellular potassium for extracellular hydrogen ion in an effort to correct the acidosis; hyperglycemia may be present, secondary to systemic release of epinephrine, glucagon, and cortisol due to hypovolemia; patients have elevated blood urea nitrogen and creatinine levels consistent with prerenal azotemia.
Medical Management
Rehydration is the first priority in the treatment of cholera. Rehydration is accomplished in 2 phases: rehydration and maintenance.
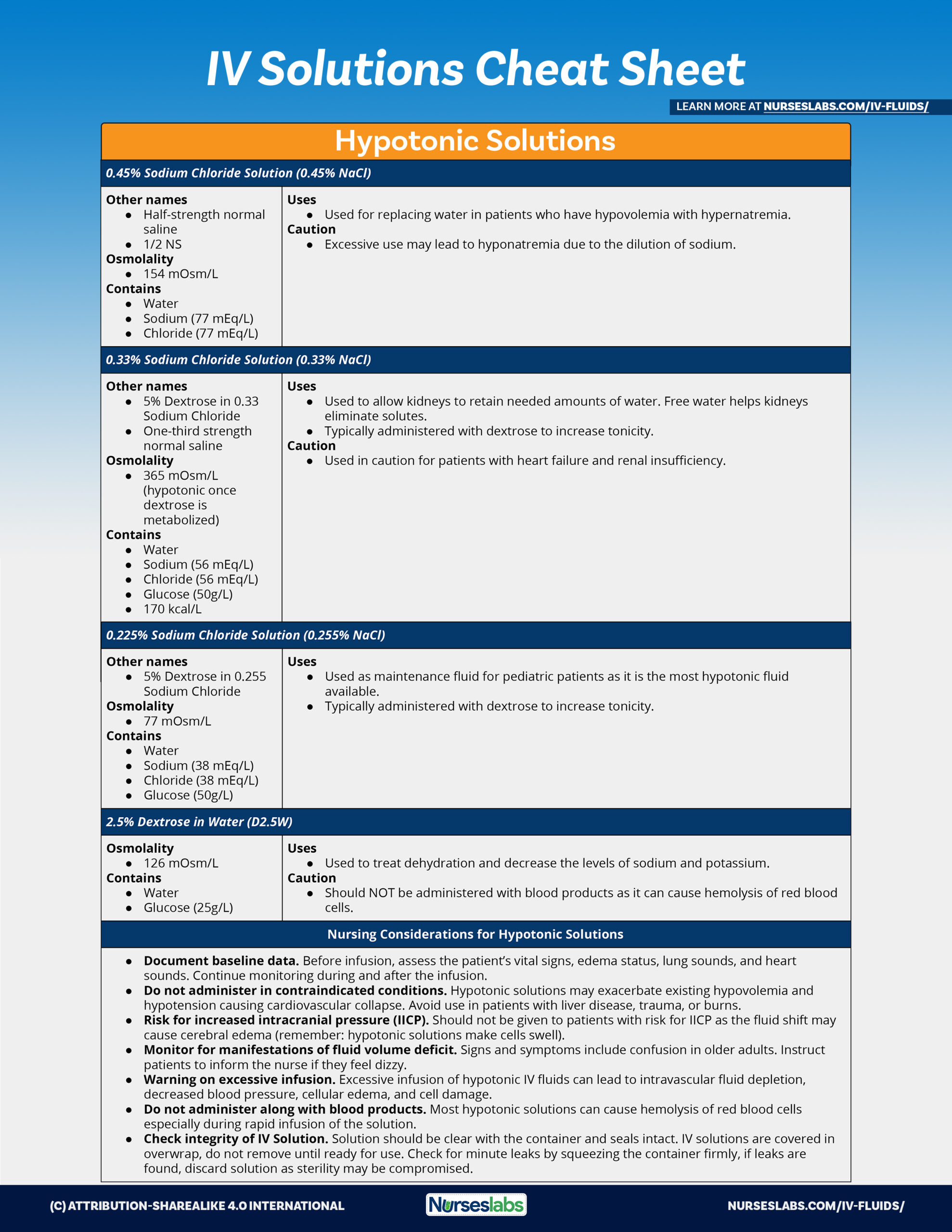
- Rehydration phase. The goal of the rehydration phase is to restore normal hydration status, which should take no more than 4 hours; set the rate of intravenous infusion in severely dehydrated patients at 50-100 mL/kg/hr; Lactated Ringer solution is preferred over isotonic sodium chloride solution because saline does not correct metabolic acidosis.
- Maintenance phase. The goal of the maintenance phase is to maintain normal hydration status by replacing ongoing losses; the oral route is preferred, and the use of oral rehydration solution (ORS) at a rate of 500-1000 mL/hr is recommended.
- Cholera cots. In areas where cholera is endemic, cholera cots have been used to assess the volume of ongoing stool losses; a cholera cot is a cot covered by a plastic sheet with a hole in the center to allow the stool to collect in a calibrated bucket underneath.
- Diet. Resume feeding with a normal diet when vomiting has stopped; continue breastfeeding infants and young children.
Pharmacological Management
Antimicrobial therapy for cholera is an adjunct to fluid therapy and is not an essential therapeutic component.
- Antibiotics. Empiric antimicrobial therapy must be comprehensive and should cover all likely pathogens in the context of the clinical setting; although not necessarily curative, treatment with an antibiotic to which the organism is susceptible diminishes the duration and volume of the fluid loss and hastens clearance of the organism from stool.
- Vaccines. In June, 2016, the first U.S. cholera vaccine was approved by the FDA; contains live attenuated cholera bacteria that replicate in the gastrointestinal tract of the recipient to provide immunity; it is indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in adults aged 18-64 y traveling to cholera-affected areas.
Nursing Management
The nursing care of a client with cholera include the following:
Nursing Assessment
Assessment of the patient with cholera are as follows:
- Assess for dehydration. Assess the status of dehydration ( skin color, temperature, skin turgor, mucous membranes, eyes, crown, body temperature, pulse, respiration, behavior, weight loss).
- Observe for diarrhea. Observe for a sudden attack of diarrhea, fever, anorexia, vomiting, nausea, abdominal cramps, increased bowel sounds, and bowel movements more than 3 times a day, with liquid stool consistency, with or without mucus or blood.
- Assess the level of knowledge of the family. Assess for the knowledge of diarrhea at home, dietary knowledge, and knowledge about the prevention of recurrent diarrhea.
Nursing Diagnosis
Based on the assessment data, the major nursing diagnosis for cholera are:
- Deficient fluid volume related to excessive fluid loss through the stool or emesis.
- Imbalanced Nutrition: less than body requirements related to loss of fluids through diarrhea, inadequate intake.
- Risk for infection related to microorganisms that penetrate the gastrointestinal tract.
- Impaired Skin Integrity: perianal, related to irritation from diarrhea.
- Anxiety related to separation from parents, unfamiliar environment, a stressful procedure.
Nursing Care Planning and Goals
The major nursing care planning goals for cholera:
- Patient will maintain adequate hydration.
- Patient will consume adequate nutritional requirements.
- Patient will prevent onset of infection.
- Patient will maintain skin integrity.
- Patient will prevent anxiety.
Nursing Interventions
The nursing interventions on a patient diagnosed with cholera are:
- Monitor intake and output. Note number, character, and amount of stools; estimate insensible fluid losses like diaphoresis; measure urine specific gravity and observe for oliguria.
- Weigh daily. Daily weight is an indicator of overall fluid and nutritional status.
- Maintain hydration. Replace ongoing fluid losses until diarrhea stops.
- Administer medications as indicated. Give an oral antibiotic to the patient with severe dehydration as prescribed.
Evaluation
Nursing goals are met as evidenced by:
- Patient was able to maintain adequate hydration.
- Patient was able to consume adequate nutritional requirements.
- Patient was able to prevent onset of infection.
- Patient was able to maintain skin integrity.
- Patient was able to prevent anxiety.
Documentation Guidelines
Documentation in a patient with cholera include the following:
- Individual findings, including factors affecting, interactions, nature of social exchanges, specifics of individual behavior.
- Cultural and religious beliefs, and expectations.
- Plan of care.
- Teaching plan.
- Responses to interventions, teaching, and actions performed.
- Attainment or progress toward the desired outcome.
Practice Quiz: Cholera
Nursing practice questions for cholera. For more practice questions, visit our NCLEX practice questions page.
Exam Mode
In Exam Mode: All questions are shown but the results, answers, and rationales (if any) will only be given after you’ve finished the quiz.
Practice Quiz: Cholera
Practice Mode
Practice Mode: This is an interactive version of the Text Mode. All questions are given in a single page and correct answers, rationales or explanations (if any) are immediately shown after you have selected an answer. No time limit for this exam.
Practice Quiz: Cholera
Text Mode
Text Mode: All questions and answers are given on a single page for reading and answering at your own pace. Be sure to grab a pen and paper to write down your answers.
1. A 90-year-old client is confined to the unit for two weeks. He has been receiving antibiotics for more than a week and tells that he is having frequent watery stools. Which action will you take first?
A. Place the client on contact precautions
B. Educate the client about correct hand washing
C. Notify the physician about the loose stools
D. Get stool specimens for culture
1. Answer: A. Place the client on contact precautions.
- Option A: Prioritization. The client may have Clostridium difficile infection based on his age, history of antibiotic therapy, and watery stools. The initial action should be to place him on contact precautions to prevent the spread of C. difficile to other clients.
- Options B, C, and D: The other actions are also necessary and should be taken after placing the client on contact precautions.
2. A client who has frequent watery stool is admitted to the unit due to dehydration. Which nursing action should the charge nurse delegate to an LPN/LVN?
A. Giving the ordered metronidazole (Flagyl) 500 mg PO to the client
B. Reconsidering the client’s medical history for any risk factors for diarrhea
C. Doing ongoing assessments to determine the client’s hydration status
D. Explaining the purpose of ordered stool cultures to the client family
2. Answer: A. Giving the ordered metronidazole (Flagyl) 500 mg PO to the client.
- Option A: Delegation. LPN/LVN scope of practice and education include administration of medications.
- Options B, C, and D: Assessment of hydration status, client and family education, and assessment of client risk factors for diarrhea should be done by the RN.
3. The nurse is caring for a 20 lbs (9 kg) 6 month-old with a 3-day history of diarrhea, occasional vomiting and fever. Peripheral intravenous therapy has been initiated, with 5% dextrose in 0.33% normal saline with 20 mEq of potassium per liter infusing at 35 ml/hr. Which finding should be reported to the healthcare provider immediately?
A. 3 episodes of vomiting in 1 hour
B. Periodic crying and irritability
C. Vigorous sucking on a pacifier
D. No measurable voiding in 4 hours
3. Answer: D. No measurable voiding in 4 hours.
- Option D: The concern is possible hyperkalemia, which could occur with continued potassium administration and a decrease in urinary output since potassium is excreted via the kidneys.
4. A 5-month old infant was brought by his mother to the health center because of diarrhea occurring 4 to 5 times a day. His skin goes back slowly after a skin pinch and his eyes are sunken. Using the IMCI guidelines, you will classify this infant in which category?
A. No signs of dehydration
B. Some dehydration
C. Severe dehydration
D. The data is insufficient
4. Answer: B. Some dehydration.
- Option B: Using the assessment guidelines of IMCI, a child (2 months to 5 years old) with diarrhea is classified as having SOME DEHYDRATION if he shows 2 or more of the following signs: restless or irritable, sunken eyes, the skin goes back slow after a skin pinch.
5. Based on the assessment, you classified a 3-month old infant with the chief complaint of diarrhea in the category of SOME DEHYDRATION. Based on the IMCI management guidelines, which of the following will you do?
A. Bring the infant to the nearest facility where IV fluids can be given
B. Supervise the mother in giving 200 to 400 ml of Oresol in 4 hours
C. Give the infant’s mother instructions on home management
D. Keep the infant in your health center for close observation
5. Answer: B. Supervise the mother in giving 200 to 400 ml. of Oresol in 4 hours.
- Option B: In the IMCI management guidelines, SOME DEHYDRATION is treated with the administration of Oresol within a period of 4 hours. The amount of Oresol is best computed on the basis of the child’s weight (75 ml/kg body weight). If the weight is unknown, the amount of Oresol is based on the child’s age.
References
Sources and references for this cholera study guide: